CDC advisers back broad rollout out of new COVID boosters
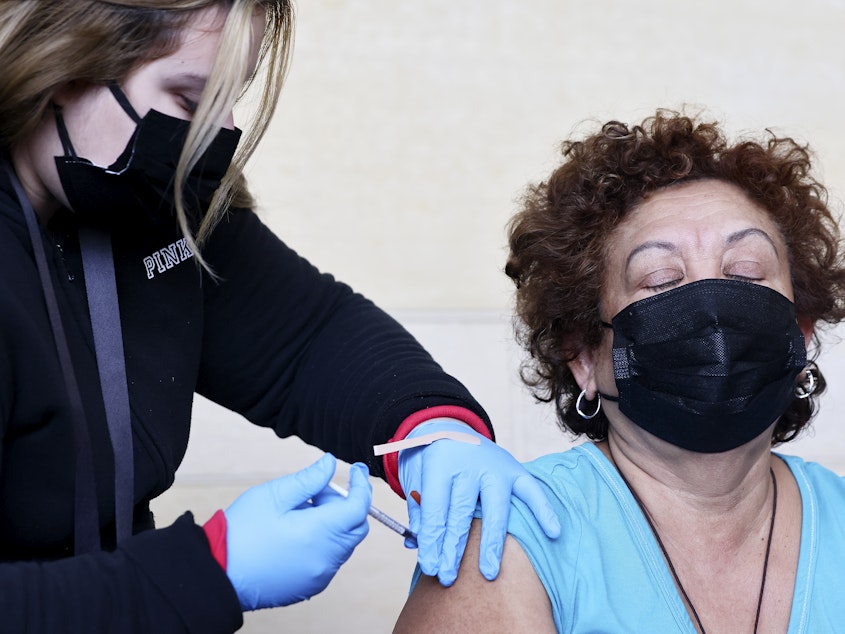
A panel of advisers to the Centers for Disease Control and Prevention backed the broad use of new COVID-19 vaccines, as cases of the respiratory illness rise.
The advisers voted 13-1 to recommend the vaccines for people ages 6 months and older. While the benefits appear to be greatest for the oldest and youngest people, the benefits of vaccination exceed the risks for everyone, according to a CDC analysis.
The universal recommendation, as opposed to one that applies to selected groups, could ease the rollout of the vaccine and improve access and equity.
"Let's keep America strong, healthy," said Dr. Camille Kotton, a panel member who voted in favor of the recommendation and who is an infectious disease specialist at Harvard Medical School. "Let's do away with COVID-19 as best we can by prevention of disease through vaccines. Let's make things clear."
The Food and Drug Administration gave the go-ahead to vaccines from Moderna and Pfizer-BioNTech Monday. A new vaccine from Novavax is under FDA review and may be approved soon.
Sponsored
The new vaccines target a much more recent variant of the omicron strain called XBB.1.5 that was selected by the FDA in June for use in formulating new vaccines. The idea, akin to how flu vaccines are made, is to match a seasonal vaccine to the virus that is infecting people.
Since the FDA's decision, other variants have overtaken XBB.1.5, but laboratory data suggest the new vaccines should provide good protection against COVID-19, including serious illness, hospitalization and death. The new shots can bolster immunity from previous vaccinations and COVID illness.
The last step before vaccination with the new shots can start is a formal decision by the CDC director. The decision is expected to quickly follow the panel's vote.
The new shots could become available as soon as Wednesday in some parts of the country. They're not technically free anymore, but for most people insurance will pay for them. The federal government will make the shots available for the uninsured at no cost. [Copyright 2023 NPR]



